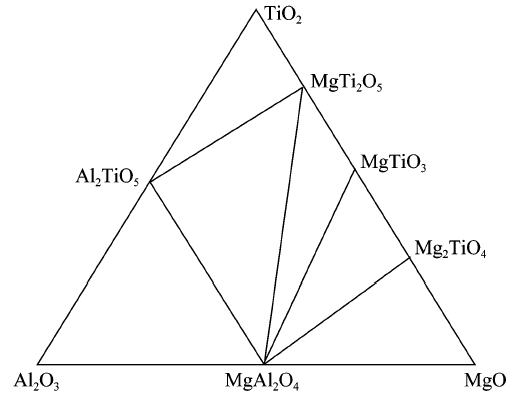
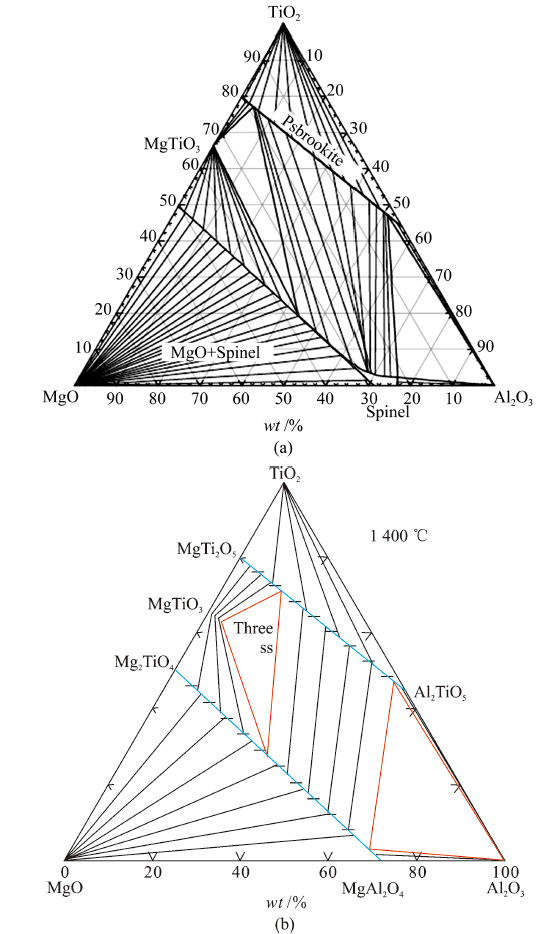
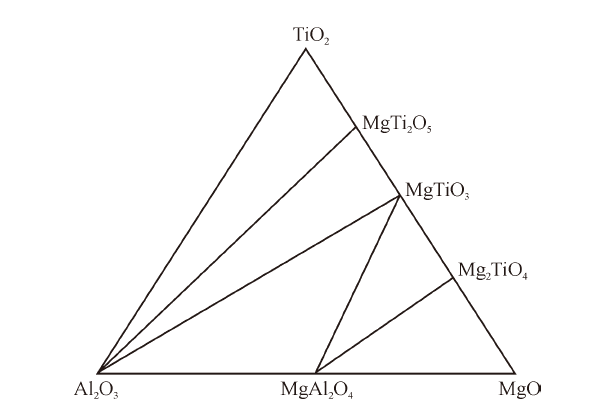
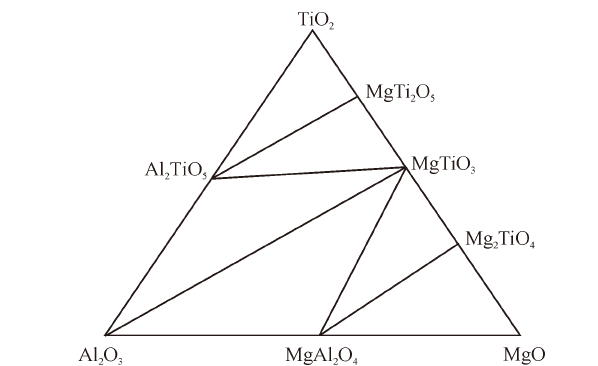
China's Refractories ›› 2021, Vol. 30 ›› Issue (3): 17-22.DOI: 10.19691/j.cnki.1004-4493.2021.03.004
Previous Articles Next Articles
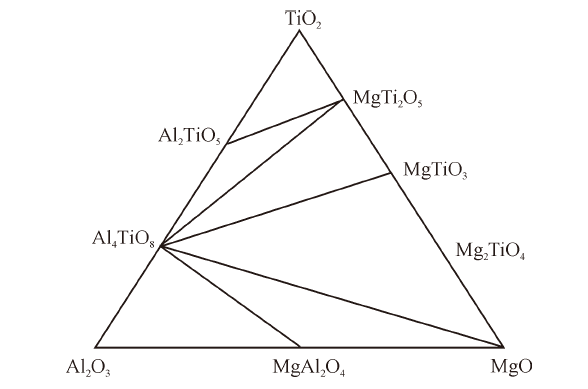
Thermodynamic Modeling of MgO-Al2O3-TiO2 System
Oksana M. BORYSENKO1,*( ), Galina M. SHABANOVA1, Sergey M. LOGVINKOV2, Lgor A. OSTAPENKO3
), Galina M. SHABANOVA1, Sergey M. LOGVINKOV2, Lgor A. OSTAPENKO3
- 1 Kharkiv Polytechnic Institute, National Technical University, Kharkiv 61002, Ukraine
2 Simon Kuznets Kharkiv National University of Economics, Kharkiv 61166, Ukraine
3 TOV “Druzhkivskiy Vognetrivkiy zavod”, Druzhkovka 84293, Ukraine
-
Online:2021-09-15Published:2021-11-26 -
Contact:Oksana M. BORYSENKO -
About author:Oksana M. Borysenko is a docent, and a doctoral candidate of the Department of Ceramics, Refractory Materials, Glass and Enamels Technology of Kharkiv Polytechnic Institute of National Technical University in Ukraine, majored in technical sciences. Her field of scientific interests embraces refractory nonmetal materials, multicomponent system condition diagrams, solid phase exchange reactions and their conjugation, and the self-organization of phases into dissipative structures. She had more than 130 scientific publications.
Cite this article
Oksana M. BORYSENKO, Galina M. SHABANOVA, Sergey M. LOGVINKOV, Lgor A. OSTAPENKO. Thermodynamic Modeling of MgO-Al2O3-TiO2 System[J]. China's Refractories, 2021, 30(3): 17-22.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.cnref.cn/EN/10.19691/j.cnki.1004-4493.2021.03.004
| Compound | -ΔH0298 K/ (kJ · mol-1) | ΔS0298 K/ [J · (mol · K)-1] | Cp = a + bТ + c'T-2 /[J · (mol · K)-1] | Reference | ||
|---|---|---|---|---|---|---|
| a | b×103 | -с×10-5 | ||||
| Al2O3 | 1 676.057 7 | 50.95 | 115.02 | 11.80 | 35.06 | [ |
| MgO | 601.241 | 26.924 | 42.59 | 7.28 | 6.19 | [ |
| TiO2 anatase | 912.53 | 34.727 | 44.225 | 15.062 | 7.782 | [ |
| TiO2 rutile | 943.492 | 49.915 | 74.6 | 2.092 | 17.698 | [ |
| MgAl2O4 | 2 297.02 | 80.58 | 153.97 | 26.78 | 40.92 | [ |
| MgTiO3 | 1 571.93 | 74.56 | 118.37 | 13.27 | 27.32 | [ |
| Mg2TiO4 | 2 163.55 | 115.10 | 154.64 | 35.73 | 28.83 | [ |
| MgTi2O5 | 2 507.89 | 138.91 | 170.21 | 38.49 | 30.75 | [ |
| Al2TiO5 | 2 607.47 | 109.62 | 182.55 | 22.18 | 46.91 | [ |
| Al4TiO8 | 4 194.883 | 211.576 | 395.84-1.1634×107×Т-2--1 925.3Т-0.5+1.505×109×Т-3 | [ | ||
Table 1 Initial thermodynamic data
| Compound | -ΔH0298 K/ (kJ · mol-1) | ΔS0298 K/ [J · (mol · K)-1] | Cp = a + bТ + c'T-2 /[J · (mol · K)-1] | Reference | ||
|---|---|---|---|---|---|---|
| a | b×103 | -с×10-5 | ||||
| Al2O3 | 1 676.057 7 | 50.95 | 115.02 | 11.80 | 35.06 | [ |
| MgO | 601.241 | 26.924 | 42.59 | 7.28 | 6.19 | [ |
| TiO2 anatase | 912.53 | 34.727 | 44.225 | 15.062 | 7.782 | [ |
| TiO2 rutile | 943.492 | 49.915 | 74.6 | 2.092 | 17.698 | [ |
| MgAl2O4 | 2 297.02 | 80.58 | 153.97 | 26.78 | 40.92 | [ |
| MgTiO3 | 1 571.93 | 74.56 | 118.37 | 13.27 | 27.32 | [ |
| Mg2TiO4 | 2 163.55 | 115.10 | 154.64 | 35.73 | 28.83 | [ |
| MgTi2O5 | 2 507.89 | 138.91 | 170.21 | 38.49 | 30.75 | [ |
| Al2TiO5 | 2 607.47 | 109.62 | 182.55 | 22.18 | 46.91 | [ |
| Al4TiO8 | 4 194.883 | 211.576 | 395.84-1.1634×107×Т-2--1 925.3Т-0.5+1.505×109×Т-3 | [ | ||
| Reaction number | ΔG /(kJ · mol-1), Temperature /K | ||||||
|---|---|---|---|---|---|---|---|
| 800 | 1 000 | 1 200 | 1 400 | 1 600 | 1 800 | 1 900 | |
| 0 | - | - | - | 0.715 | -0.558 | -1.640 | -2.094 |
| 1** | - | - | - | 4.459 | 4.025 | 3.129 | 2.484 |
| 2** | - | - | - | - | - | -129.484 | -145.566 |
| 3* | -106.866 | -124.409 | -143.450 | -163.683 | - | - | - |
| 3** | - | - | - | -0.702 | -0.308 | -0.228 | -0.322 |
| 4** | - | - | - | 0.013 | -0.867 | -1.868 | -2.416 |
| 5** | - | - | - | - | - | -139.481 | -155.370 |
| 6* | -57.037 | -66.189 | -76.144 | -86.639 | - | - | - |
| 6** | - | - | - | -5.149 | -5.102 | -5.226 | -5.223 |
| 7** | - | - | - | 19.675 | 15.322 | 10.427 | 7.849 |
| 8** | - | - | - | - | - | -125.271 | -142.687 |
| 9* | -35.824 | -47.031 | -59.216 | -72.140 | - | - | - |
| 9** | - | - | - | 9.349 | 6.653 | 3.756 | 2.234 |
| 10** | - | - | - | 29.314 | 29.667 | 30.088 | 30.320 |
| 11** | - | - | - | - | - | -75.568 | -89.896 |
| 12 | - | - | - | -11.027 | -8.978 | -6.944 | -5.936 |
| 13 | - | - | - | -10.312 | -9.536 | -8.584 | -8.031 |
| 14 | - | - | - | 4.187 | 2.318 | 0.398 | -0.572 |
| 15 | - | - | - | - | - | 257.828 | 289.522 |
| 16 | - | - | - | - | - | 123.801 | 139.698 |
| 17 | - | - | - | - | - | 132.784 | 147.157 |
| 18** | - | - | - | 3.054 | 3.407 | 2.673 | 1.840 |
| 19 | -21.213 | -19.157 | -16.928 | -14.499 | -11.855 | -8.982 | -7.458 |
| 20 | -35.218 | -30.345 | -25.017 | -19.402 | -13.615 | -7.740 | -4.791 |
| 21 | - | - | - | - | - | -404.102 | -229.125 |
| 22 | - | - | - | - | - | -273.356 | -303.490 |
| 23 | - | - | - | - | - | -396.760 | -443.761 |
| 24 | - | - | - | - | - | -105.656 | -120.216 |
| 25 | - | - | - | - | - | -140.464 | -174.482 |
Table 2 Calculated change in Gibbs free energy value for reactions of Al2O3-MgO-TiO2
| Reaction number | ΔG /(kJ · mol-1), Temperature /K | ||||||
|---|---|---|---|---|---|---|---|
| 800 | 1 000 | 1 200 | 1 400 | 1 600 | 1 800 | 1 900 | |
| 0 | - | - | - | 0.715 | -0.558 | -1.640 | -2.094 |
| 1** | - | - | - | 4.459 | 4.025 | 3.129 | 2.484 |
| 2** | - | - | - | - | - | -129.484 | -145.566 |
| 3* | -106.866 | -124.409 | -143.450 | -163.683 | - | - | - |
| 3** | - | - | - | -0.702 | -0.308 | -0.228 | -0.322 |
| 4** | - | - | - | 0.013 | -0.867 | -1.868 | -2.416 |
| 5** | - | - | - | - | - | -139.481 | -155.370 |
| 6* | -57.037 | -66.189 | -76.144 | -86.639 | - | - | - |
| 6** | - | - | - | -5.149 | -5.102 | -5.226 | -5.223 |
| 7** | - | - | - | 19.675 | 15.322 | 10.427 | 7.849 |
| 8** | - | - | - | - | - | -125.271 | -142.687 |
| 9* | -35.824 | -47.031 | -59.216 | -72.140 | - | - | - |
| 9** | - | - | - | 9.349 | 6.653 | 3.756 | 2.234 |
| 10** | - | - | - | 29.314 | 29.667 | 30.088 | 30.320 |
| 11** | - | - | - | - | - | -75.568 | -89.896 |
| 12 | - | - | - | -11.027 | -8.978 | -6.944 | -5.936 |
| 13 | - | - | - | -10.312 | -9.536 | -8.584 | -8.031 |
| 14 | - | - | - | 4.187 | 2.318 | 0.398 | -0.572 |
| 15 | - | - | - | - | - | 257.828 | 289.522 |
| 16 | - | - | - | - | - | 123.801 | 139.698 |
| 17 | - | - | - | - | - | 132.784 | 147.157 |
| 18** | - | - | - | 3.054 | 3.407 | 2.673 | 1.840 |
| 19 | -21.213 | -19.157 | -16.928 | -14.499 | -11.855 | -8.982 | -7.458 |
| 20 | -35.218 | -30.345 | -25.017 | -19.402 | -13.615 | -7.740 | -4.791 |
| 21 | - | - | - | - | - | -404.102 | -229.125 |
| 22 | - | - | - | - | - | -273.356 | -303.490 |
| 23 | - | - | - | - | - | -396.760 | -443.761 |
| 24 | - | - | - | - | - | -105.656 | -120.216 |
| 25 | - | - | - | - | - | -140.464 | -174.482 |
| [1] |
Kirill S. Shnurenko, Andrey P. Shevchik, Vladimir V. Kozlov, Lev A. Lebedev. Effect of addition of nanostructured alumomagnesium spinel obtained by SHS-method on physicomechanical properties of spinel ceramics. Bulletin of the Saint Petersburg State Institute of Technology (Technical University), 2018, 44(70):17-20.
DOI URL |
| [2] | A. N. Travitskova. VIII research and practice conference “Essential issues of the refractory engineering”. Novye Ogneupory (New refra-ctories), 2018, 3:69-76. |
| [3] | A. S. Suvorov, A. V. Rusinov, V. N. Fishchev, N. V. Alekseeva. High-temperature materials with a low integral coefficient of thermal expansion. Refractories and Technical Ceramics, 2008, 2:11-17. |
| [4] |
H. J. Seifert, A. Kussmaul, F. Aldinger. Phase equilibria and diffusion paths in the Ti-Al-O-N system. Journal of Alloys and Compounds, 2001, 317-318(12):19-25.
DOI URL |
| [5] |
In-Ho Jung, Gunnar Eriksson, Ping Wu, Arthur Pelton. Thermodynamic modeling of the Al2O3-Ti2O3-TiO2 system and its applications to the Fe-Al-Ti-O inclusion diagram. ISIJ International, 2009, 49(9):1290-1297.
DOI URL |
| [6] | Yoshikazu Suzuki, Yutaka Shinoda. Magnesium dititanate (MgTi2O5) with pseudobrookite structure: a review. Science and Technology of Advanced Materials, 2011, 12(3). |
| [7] | Sharon Samyuktha, T. Subba Raob, R. Padma Suvarna. Synthesis and dielectric properties of MgTiO3 ceramic material. International Journal of Engineering and Technical Research, 2016, 5(5):245-249. |
| [8] |
Yuan-Bin Chen. Dielectric properties and crystal structure of Mg2TiO4 ceramics substituting Mg2+ with Zn2+ and Co2+. Journal of Alloys and Compounds, 2012, 513(5):481-486.
DOI URL |
| [9] | A. S. Berezhnoy. Multicomponent systems of oxides. Kiev: “Naukova Dumka”, 1970: 544. |
| [10] |
Tatjana Jantzen, Klaus Hack, Elena Yazhenskikh, Michael Müller. Addition of TiO2 and Ti2O3 to the Al2O3-FeO-Fe2O3-MgO system. Calphad, 2018, 62:187-200.
DOI URL |
| [11] | Malcolm W. Chase, Jr. NIST-JANAF Thermochemical Tables, fourth edition. Part I, Al-Co. Access mode: https://janaf.nist.gov/pdf/JANAF-FourthEd-1998-1Vol1-Intro.pdf. |
| [12] |
S. M. Logvinkov, G. D. Semchenko, D. A. Kobyzev, V. I. Babushkin. Thermodynamics of phase relations in the subsolidus of the MgO-Al2O3-SiO2 system. Refractories and Industrial Ceramics, 2001, 42:434-439.
DOI URL |
| [13] | V. I. Babushkin, G. M. Matveyey, O. P. Mchedlov-Petrosyan. Thermodynamics of silicates. Moscow: Stroyizdat, 1985: 408. |
| [14] | Yu. D. Tretyakov. Solid-phase reactions. Moscow: Chemistry, 1978: 360. |
| [1] | ZHOU Lianzhuo, WANG Zhoufu, WANG Xitang, LIU Hao, MA Yan, QUAN Zhenghuang. Effect of Al Powder and Si Powder Additions on Structure and Properties of Unburned Magnesium Aluminate Spinel Refractories [J]. China's Refractories, 2023, 32(3): 14-19. |
| [2] | HAO Xian, LIU Guoqi, LI Zhixing, XU Chaojie, ZHANG Jianwei, LI Yong, LI Hongyu, LI Hongxia, FU Baoquan. Effect of Al-Si Alloy Addition on Properties of Fused Spinel Carbon Materials [J]. China's Refractories, 2022, 31(4): 45-52. |
| [3] | Oksana M. BORYSENKO, Sergey M. LOGVINKOV, Galina M. SHABANOVA, Andrii A. IVASHURA, Alla M. KOROHODSKA. Thermodynamics of Phase Equilibria in FeO-TiO2-Al2O3 System [J]. China's Refractories, 2022, 31(2): 40-44. |
| [4] | ZHAO Huizhong, LI Jingjie. Effect of Spinels on Properties of MgO-Al2O3 Refractories for RH Snorkels [J]. China's Refractories, 2021, 30(3): 1-6. |
| [5] | GAO Zhenxin. Phase Relationship at Subsolidus Tem-perature of MgO-MgAl2O4-ZrO2 Subsystem [J]. China's Refractories, 2020, 29(1): 1-6. |
| [6] | CHEN Ding*, GU Huazhi, HUANG Ao, XIE Jing. Research on Alkali Resistance of Alumina-rich MgAl2O4 Spinel for Alkali Recovery Furnace [J]. , 2018, 27(2): 37-40. |
| [7] | LIU Jiangbo, WANG Zhoufu, LIU Hao, WANG Xitang, MA Yan. Effect of Y2O3 Addition on Sintering Properties and Microstructure of Spinel Materials [J]. , 2018, 27(1): 39-43. |
| [8] | Michail I. RYSCHENKO,Yaroslav N. PITAK,Elena Yu. FEDORENKO, Maria Yu. LISYUTKINA, Alexey V. SHEVTSOV. Subsolidus Conceptual Design of CaO-Al2O3-TiO2-SiO2 System and Its Significance for Manufacturing Advanced Ceramics [J]. , 2016, 25(1): 44-52. |
| [9] | CHEN Yang, WU Hongping, ZHU Dongdong, ZHANG Hang. Development of Magnesium Aluminate Spinel Castable for Roof of EAF [J]. , 2014, 23(2): 27-29. |
| [10] | KE Changming, GAN Lin. Synthesis of MgAl2O4 Powder in KCl-LiCl-KF System Molten Salt [J]. China's Refractories, 2011, 20(2): -. |
| [11] | BI Wanli, ZHANG Guodong, LUO Xudong. Synthesis of Magnesium Aluminate Spinel-corundum Composite with Waste Slide Plate and Magnesia Carbon Brick [J]. China's Refractories, 2011, 20(2): -. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||