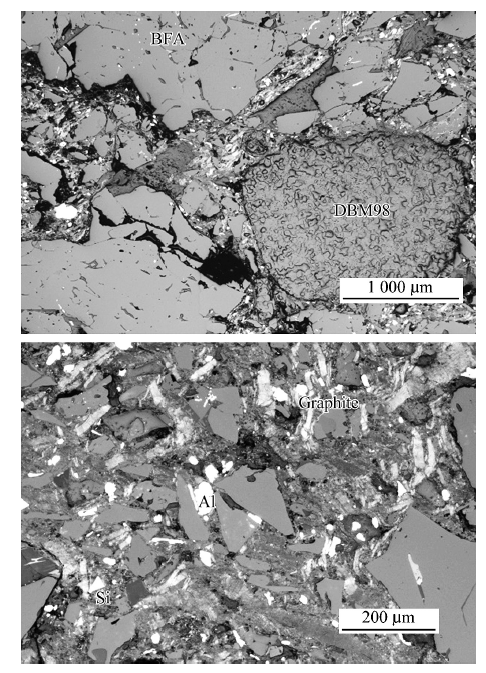
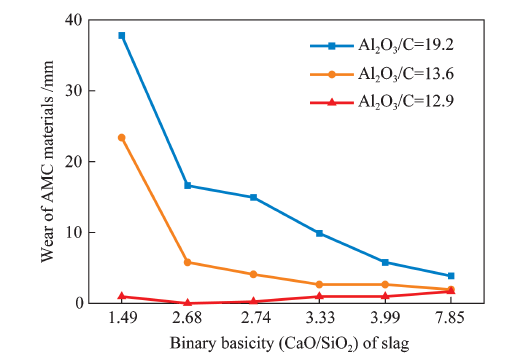
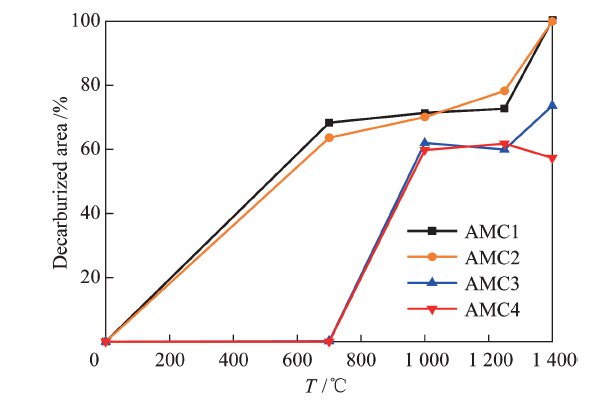
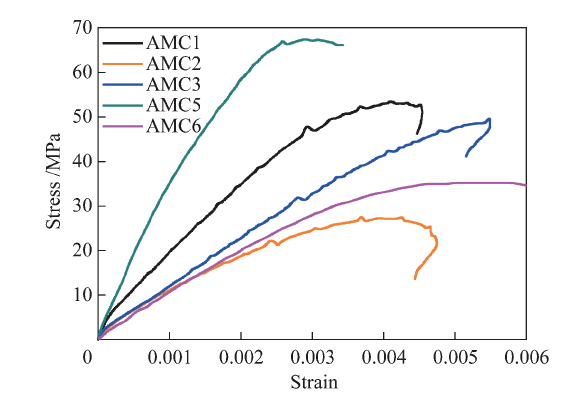
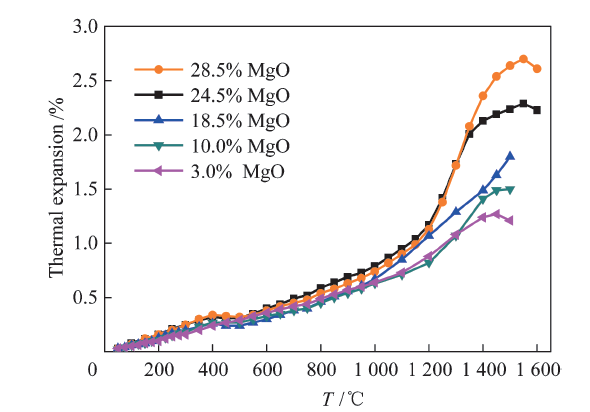
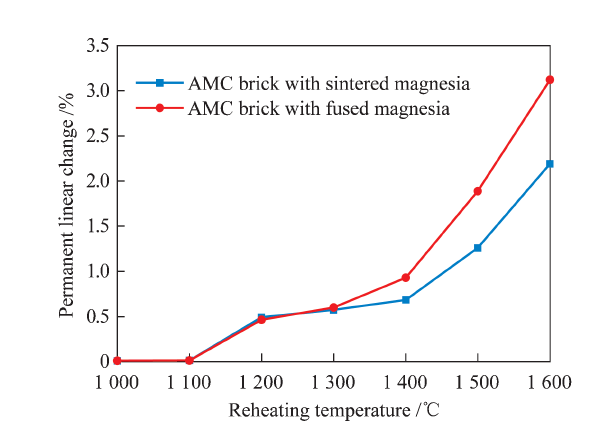
China's Refractories ›› 2021, Vol. 30 ›› Issue (1): 41-48.DOI: 10.19691/j.cnki.1004-4493.2021.01.007
• Original article • Previous Articles
A Retrospective Review of Alumina-magnesia-carbon Refractories
GUO Zongqi1,*( ), ZAMBONI Stefano1, GAO Jianying2, GAN Feifang3
), ZAMBONI Stefano1, GAO Jianying2, GAN Feifang3
- 1 Trasteel International SA, Lugano CH-6900, Switzerland
2 Imerys (China) Co., Ltd., Tianjin 300457, China
3 Baoshan Iron & Steel Co., Ltd., Shanghai 201900, China
-
Online:2021-03-15Published:2021-05-01 -
Contact:GUO Zongqi -
About author:Dr. Guo Zongqi is interested in the application and research of spinel technologies in burnt basic brick and unburnt refractory brick. In recent years, He focuses on practices of the mismatch of thermal expansion of MA and FA spinel in magnesia refractories, as well as monolization function of in-situ spinel in alumina-based brick for ladle lining.
Cite this article
GUO Zongqi, ZAMBONI Stefano, GAO Jianying, GAN Feifang. A Retrospective Review of Alumina-magnesia-carbon Refractories[J]. China's Refractories, 2021, 30(1): 41-48.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.cnref.cn/EN/10.19691/j.cnki.1004-4493.2021.01.007
| Raw material | TA | WFA | BFA | Bauxite | DBM98 | DBM97 | FM97.5 |
|---|---|---|---|---|---|---|---|
| Al2O3 /mass% | 99.5 | 99.50 | 95.50 | 89.00 | 0.07 | 0.15 | 0.10 |
| MgO /mass% | 98.50 | 97.10 | 97.50 | ||||
| SiO2 /mass% | 0.09 | 0.10 | 1.30 | 4.00 | 0.10 | 0.65 | 0.60 |
| Fe2O3 /mass% | 0.02 | 0.05 | 0.10 | 1.50 | 0.48 | 0.80 | 0.60 |
| TiO2 /mass% | 2.50 | 3.50 | |||||
| Na2O + K2O /mass% | 0.40 | 0.20 | 0.10 | 0.30 | |||
| CaO /mass% | 0.50 | 0.84 | 1.30 | 1.20 | |||
| Bulk density /(g · cm-3) | 3.50 | 3.69 | 3.90 | 3.28 | 3.43 | 3.28 | 3.50 |
Table 1 Chemical and physical analyses of main raw materials for AMC bricks
| Raw material | TA | WFA | BFA | Bauxite | DBM98 | DBM97 | FM97.5 |
|---|---|---|---|---|---|---|---|
| Al2O3 /mass% | 99.5 | 99.50 | 95.50 | 89.00 | 0.07 | 0.15 | 0.10 |
| MgO /mass% | 98.50 | 97.10 | 97.50 | ||||
| SiO2 /mass% | 0.09 | 0.10 | 1.30 | 4.00 | 0.10 | 0.65 | 0.60 |
| Fe2O3 /mass% | 0.02 | 0.05 | 0.10 | 1.50 | 0.48 | 0.80 | 0.60 |
| TiO2 /mass% | 2.50 | 3.50 | |||||
| Na2O + K2O /mass% | 0.40 | 0.20 | 0.10 | 0.30 | |||
| CaO /mass% | 0.50 | 0.84 | 1.30 | 1.20 | |||
| Bulk density /(g · cm-3) | 3.50 | 3.69 | 3.90 | 3.28 | 3.43 | 3.28 | 3.50 |
| Physical properties | Or.1 | Or.2 | Or.3 | Or.4 |
|---|---|---|---|---|
| Tempering at 200 °C for 8-10 h | ||||
| Apparent porosity /% | 3.2 | 3.8 | 4.2 | 5.1 |
| Bulk density /(g · cm-3) | 3.10 | 3.12 | 3.12 | 3.15 |
| Cold crushing strength /MPa | 76.2 | 82.7 | 85.6 | 90.0 |
| Coking at 1 100 °C for 2 h | ||||
| Apparent porosity /% | 8.2 | 9.6 | 9.1 | 10.2 |
| Bulk density /(g · cm-3) | 3.03 | 3.04 | 3.04 | 3.07 |
| Residual carbon /% | 8.2 | 6.2 | 4.5 | 3.2 |
| Thermal conductivity at 1 000 °C/[W · (m · K)-1] | 9.21 | 7.26 | 5.26 | 3.92 |
| Spalling index | 100 | 125 | 130 | 150 |
| Heating at 1 600 °C for 2 h, permanent linear change (PLC) | ||||
| PLC after first heating /% | +1.90 | +1.72 | +1.93 | +2.29 |
| PLC after second heating /% | +1.73 | +1.62 | +1.91 | +2.21 |
| PLC after third heating /% | +1.51 | +1.52 | +1.85 | +2.10 |
Table 2 Influence of graphite addition on thermal conductivity of AMC bricks
| Physical properties | Or.1 | Or.2 | Or.3 | Or.4 |
|---|---|---|---|---|
| Tempering at 200 °C for 8-10 h | ||||
| Apparent porosity /% | 3.2 | 3.8 | 4.2 | 5.1 |
| Bulk density /(g · cm-3) | 3.10 | 3.12 | 3.12 | 3.15 |
| Cold crushing strength /MPa | 76.2 | 82.7 | 85.6 | 90.0 |
| Coking at 1 100 °C for 2 h | ||||
| Apparent porosity /% | 8.2 | 9.6 | 9.1 | 10.2 |
| Bulk density /(g · cm-3) | 3.03 | 3.04 | 3.04 | 3.07 |
| Residual carbon /% | 8.2 | 6.2 | 4.5 | 3.2 |
| Thermal conductivity at 1 000 °C/[W · (m · K)-1] | 9.21 | 7.26 | 5.26 | 3.92 |
| Spalling index | 100 | 125 | 130 | 150 |
| Heating at 1 600 °C for 2 h, permanent linear change (PLC) | ||||
| PLC after first heating /% | +1.90 | +1.72 | +1.93 | +2.29 |
| PLC after second heating /% | +1.73 | +1.62 | +1.91 | +2.21 |
| PLC after third heating /% | +1.51 | +1.52 | +1.85 | +2.10 |
| Process | DTA Peak | AMC-t | AMC-b |
|---|---|---|---|
| Resin transformation | Exothermic | 392 °C | 354 °C |
| Resin transformation | Exothermic | 507 °C | 392 °C |
| Resin transformation | Exothermic | 533 °C | |
| Resin transformation, glassy-carbon oxidation | Exothermic | 630 °C | |
| Aluminum melting | Endothermic | 651 °C | 664 °C |
| Graphite oxidation | Exothermic | 914 °C | 982 °C |
| Formation of Al4C3 (Al/Al2O3 + C) | Exothermic | 1 050 °C | 1 046 °C |
| Formation of Al2O3 | Exothermic | 1 178 °C | 1 145 °C |
| Formation of MgO · Al2O3 (Al2O3/Al(l) + MgO/Mg(g)) | Exothermic | 1 178 °C | 1 145 °C |
| Formation of Al4O4C | Exothermic | 1 310 °C | 1 288 °C |
Table 3 Exothermic and endothermic peaks in AMCs’DTA
| Process | DTA Peak | AMC-t | AMC-b |
|---|---|---|---|
| Resin transformation | Exothermic | 392 °C | 354 °C |
| Resin transformation | Exothermic | 507 °C | 392 °C |
| Resin transformation | Exothermic | 533 °C | |
| Resin transformation, glassy-carbon oxidation | Exothermic | 630 °C | |
| Aluminum melting | Endothermic | 651 °C | 664 °C |
| Graphite oxidation | Exothermic | 914 °C | 982 °C |
| Formation of Al4C3 (Al/Al2O3 + C) | Exothermic | 1 050 °C | 1 046 °C |
| Formation of Al2O3 | Exothermic | 1 178 °C | 1 145 °C |
| Formation of MgO · Al2O3 (Al2O3/Al(l) + MgO/Mg(g)) | Exothermic | 1 178 °C | 1 145 °C |
| Formation of Al4O4C | Exothermic | 1 310 °C | 1 288 °C |
| Brick No. | E /GPa | σf /MPa | εf | σY /σf /% |
|---|---|---|---|---|
| AMC1 | 23 ± 10 | 52 ± 2 | 0.3 ± 0.1 | 68 ± 4 |
| AMC2 | 11 ± 2 | 25 ± 2 | 0.3 ± 0.1 | 63 ± 15 |
| AMC3 | 9 ± 2 | 46 ± 9 | 0.6 ± 0.1 | 65.4 ± 0.2 |
| AMC5 | 32 ± 14 | 58 ± 12 | 0.30 ± 0.01 | 31 ± 9 |
| AMC6 | 12 ± 5 | 38 ± 3 | 0.60 ± 0.05 | 70 ± 9 |
Table 4 Mechanical parameters of AMC refractories
| Brick No. | E /GPa | σf /MPa | εf | σY /σf /% |
|---|---|---|---|---|
| AMC1 | 23 ± 10 | 52 ± 2 | 0.3 ± 0.1 | 68 ± 4 |
| AMC2 | 11 ± 2 | 25 ± 2 | 0.3 ± 0.1 | 63 ± 15 |
| AMC3 | 9 ± 2 | 46 ± 9 | 0.6 ± 0.1 | 65.4 ± 0.2 |
| AMC5 | 32 ± 14 | 58 ± 12 | 0.30 ± 0.01 | 31 ± 9 |
| AMC6 | 12 ± 5 | 38 ± 3 | 0.60 ± 0.05 | 70 ± 9 |
| Raw materials and physical properties | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| Grain size of 10% magnesia /mm | 1-3.35 | 0-1 | <0.045, 0-1 | <0.045 |
| Fused alumina /mass% | 81.5 | 81.5 | 81.5 | 81.5 |
| Natural flake graphite /mass% | 7 | 7 | 7 | 7 |
| Novolac resin + hexa. /mass% | +3 | +3 | +3 | +3 |
| Additive /mass% | 1.5 | 1.5 | 1.5 | 1.5 |
| Apparent porosity (AP) /% | 7.43 | 7.65 | 7.84 | 7.98 |
| Bulk density (BD) /(g · cm-3) | 3.30 | 3.31 | 3.32 | 3.31 |
| AP, coked at 1 600 °C /% | 16.6 | 15.6 | 16.3 | 17.1 |
| BD, coked at 1 600 °C /(g · cm-3) | 3.00 | 3.04 | 3.02 | 2.97 |
| Primary PLC, 1 600 °C /% | +2.36 | +2.44 | +3.27 | +4.00 |
| Secondary PLC, 1 600 °C /% | +0.11 | +0.06 | +0.16 | -0.02 |
| HMOR at 1 400 °C /MPa | 4.5 | 6.9 | 7.4 | 8.2 |
Table 5 AMC refractories with different fractions of magnesia
| Raw materials and physical properties | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| Grain size of 10% magnesia /mm | 1-3.35 | 0-1 | <0.045, 0-1 | <0.045 |
| Fused alumina /mass% | 81.5 | 81.5 | 81.5 | 81.5 |
| Natural flake graphite /mass% | 7 | 7 | 7 | 7 |
| Novolac resin + hexa. /mass% | +3 | +3 | +3 | +3 |
| Additive /mass% | 1.5 | 1.5 | 1.5 | 1.5 |
| Apparent porosity (AP) /% | 7.43 | 7.65 | 7.84 | 7.98 |
| Bulk density (BD) /(g · cm-3) | 3.30 | 3.31 | 3.32 | 3.31 |
| AP, coked at 1 600 °C /% | 16.6 | 15.6 | 16.3 | 17.1 |
| BD, coked at 1 600 °C /(g · cm-3) | 3.00 | 3.04 | 3.02 | 2.97 |
| Primary PLC, 1 600 °C /% | +2.36 | +2.44 | +3.27 | +4.00 |
| Secondary PLC, 1 600 °C /% | +0.11 | +0.06 | +0.16 | -0.02 |
| HMOR at 1 400 °C /MPa | 4.5 | 6.9 | 7.4 | 8.2 |
| [1] | Mareyasu Kamiide, Shinsuke Yamamoto, Kenzo Yamamoto, Koichiro Nakahara, Naofumi Kido. Damage of Al2O3-MgO-C brick for ladle furnace. Taikabutsu Overseas, 2001,21(4):252-257. |
| [2] | Paul Williams, Ann Ha. Mineralogical studies of alumina-magnesia-carbon steel ladle refractories. Proceedings of UNITECR 1997, New Orleans, USA, 1997: 193-192. |
| [3] | S. Mukhopadhaya, R. Eswaran, V. Bhatnagar. Design aspect of a low thermal conductivity Al2O3-MgO-C brick for steel ladle. Proceedings of UNITECR 2005, Orlando, USA, 2005: 102. |
| [4] | B. Rand, B. McEnaney. Carbon binders from polymetric resin and pitch. 1. pyrolysis behavior and structure of the carbons. Transaction and Journal of The British Ceramic, 1985,84(5):157-165. |
| [5] | Vanesa Muñoz, Analía Gladys Tomba Martinez. Thermal evolution of Al2O3-MgO-C refractories. Procedia Materials Science, 2012,1:410-417. |
| [6] | Juergen Poetschke, Thomas Deinet, G Routschka, R Simmat. Properties and corrosion of AMC-refractories. Part I: characterization and oxidation. Proceedings of UNITECR 2003, Osaka, Japan 2003: 580-583. |
| [7] | Susumu Okushima, Tetsuo Sato, Kenji Yamamoto, Yutaka Nakano. Improvement of slag resistance of Al2O3-MgO-C brick. Taikabutsu Overseas, 1990,10(3):142-143. |
| [8] | W. S. Resende, R. M. Stoll, S. M. Justus, R. M. Andrade, E. Longo, J. B. Baldo, E. R. Leite, C. A. Paskocimas, L. E. B, Soledade, J. E. Gomes, J. A. Varela. Key features of alumina-magnesia-graphite refractories for steel ladle lining. Journal of the European Ceramic Society, 2000,20(9):1419-1427. |
| [9] | W. A. Calvo, P. Ortega, M. J. Velasco, V. Muñoz, P. Pena, A. G, Tomba Martinez. Characterization of alumina-magnesia-carbon refractory bricks containing aluminum and silicon. Ceramics International, 2018,44(8):8842-8855. |
| [10] | Vanesa Muñoz, Pablo G. Galliano, Elena Brandaleze, Analía G. Tomba Martinez. Chemical wear of Al2O3-MgO-C bricks by air and basic slag. Journal of the European Ceramic Society, 2015,35(5):1621-1635. |
| [11] | Vanesa Muñoz, Analía Gladys, Tomba Martinez. Thermomechanical behavior of Al2O3-MgO-C refractories under non-oxidizing atmosphere. Ceramics International, 2015,41(3, part A,): 3438-3448. |
| [12] | Akira Watanabe, Hirokuni Takahashi, Shigeyuki Takanaga, Nobuo Goto, Osamu Matsuura, Syoji Yoshida. Thermal and mechanical properties of Al2O3-MgO-C bricks. Taikabutsu Overseas, 1990,10(3):137-141. |
| [13] | Vanesa Muñoz, Pilar Pena, Analía Gladys, Tomba Martínez. Physical, chemical and thermal characterization of alumina-magnesia-carbon refractories. Ceramics International, 2014,40(7 part A):9133-9149. |
| [14] | Leonardo Musante, Vanesa Muñoz, Marcelo H. Labadie. High temperature mechanical behavior of Al2O3-MgO-C refractories for steel-making use. Ceramics International, 2011,37(5):1473-1483. |
| [15] | H. S. Tripathi, B. Mukherjee, S. Das, M. K. Haldar, S. K. Das, A. Ghosh. Synthesis and densification of magnesium aluminate spinel: effect of MgO reactivity. Ceramics International, 2003,29(8):915-918. |
| [16] | Ozan Uylas, Asim Bilge, Oezkan Kurukavak, Nafiz Oezdenir. Effect of different magnesia raw materials on spinel formation and performance of AMC bricks. Proceedings of 61th International Colloquium on Refractories, Aachen, Germany, 2018: 64-66. |
| [17] | David E. Shewmon, William N. Porter. Improving the structural integrity of steel ladle refractory linings. Proceedings of UNITECR 2005, Orlando, USA, 2005: 147. |
| [18] | S. Mukhopadhyay, A. K. Chattopadhyay, S. Adak, G. C. Das, S. K. Maitra. Characterization of expansive behaviour of Al2O3-MgO-C refractory due to in-situ spinel formation. Proceedings of UNITECR 2011, Kyoto, Japan, 2011: 31-B-7. |
| [19] | Abhinav Srivastava, Nayan Kr. Debnath, Vijay Kumar, P Hemanth Kumar, Vinay Kumar Singh. The Effect of mechanochemically activated MgO in Al2O3-MgO-C refractory. Part I: formulation and properties. Ceramics International, 2016,42(8):10116-10121. |
| [20] | Ali Baghaei, Homeyra Heydari Boroujeni, Mehdi Naeemi. Impact of magnesia grain size on the in situ spinel formation in Al2O3-MgO-C refractories. Refractories Worldforum, 2018,10(2):80-85. |
| [21] | Marcus Emmel, Christo G. Aneziris, Franscesco Sponza, Steffen Dudczig, Paolo Colonbo. In situ spinel formation in Al2O3-MgO-C filter materials for steel melt filtration. Ceramic International, 2014,40(8):13507-13513. |
| [22] | Santanu Mukhopadhyay, Arup K. Chattopadhyay, Gopesh C. Das, Saikat Maitra. Effect of MgO grain size on thermal expansion behavior of alumina-magnesia-carbon refractory. International Journal of Applied Ceramic Technology, 2014,11(6):1012-1019. |
| [1] | LIU Guoqi, WANG Minghui, LI Hongxia, YU Jianbin, GAN Feifang, GU Qiang, MA Weikui. In-situ Formation of MgAl2O4: A Methodof Tailoring Microstructure and Propertiesfor Development of High-performance Refractories [J]. China's Refractories, 2023, 32(2): 1-6. |
| [2] | ZENG Fanbo, HUANG Ao, WANG Xinlian, LI Shenghao, ZHANG Shuzhe, QU Pengcheng, GU Huazhi. Effect of CaO/SiO2 Slag Mass Ratio on Dissolution Rate of Alumina-based Refractory Ceramics [J]. China's Refractories, 2023, 32(1): 30-34. |
| [3] | GAO Jianying, ZHANG Ziying, BRUNO Touzo. Thermo-mechanical Properties of In-situ Formed Al2O3-SiC-SiAlON Composites [J]. , 2016, 25(2): 1-6. |
| [4] | HUANG Yalei,GUQiang,WENYubin, LIU Zhifang, LIU Xinhong. Thermo-mechanical Properties of In-situ Formed Al2O3-SiC-SiAlON Composites [J]. , 2016, 25(2): 48-52. |
| [5] | LIANG Feng, LI Nan, LIU Baikuan, HE Zhongyang. Effect of Carbon Source on Crystal Morphology of SiC in-situ Formed in Al2O3-Si-C Matrix [J]. , 2015, 24(2): 11-15. |
| [6] | XIE Zhaohui, CHEN Liugang, ZHAI Pengtao, ZHANG Yang. Effect of ZnO on Oxidation Resistance of Low Carbon MgO-C Refractories [J]. , 2014, 23(2): 7-11. |
| [7] | YANG Daoyuan, ZHU Kai, WU Juan. Determination Method of Thermal Expansion Coefficient of Cordierite [J]. , 2013, 22(4): 14-17. |
| [8] | Andreas BUHR,Marion SCHNABEL,LONG Bin, Reinhard EXENBERGER, Christian RAMPITSCH, Jerry DUTTON. Spinel Castables for Steel Ladle Lining: In-situ Versus Preformed [J]. China's Refractories, 2012, 21(3): -. |
| [9] | ZHANG Qiaoyan,ZHU Boquan,LI Xiangcheng. Effects of Magnesia Fines Addition and Spinel with Different Compositions on Thermal Expansion Behavior of Alumina Magnesia Castables [J]. China's Refractories, 2012, 21(2): -. |
| [10] | ZHANG Wei, DAI Wenyong, YU Xinfeng. Effect of Heat Treatment Temperature on Properties of Chinese Calcined Flint Clay Based Plastic Refractories [J]. China's Refractories, 2009, 18(2): -. |
| [11] | LI Xinjian, TANG Kun, LIU Zhongjiang. Development of Reproducing Alumina-Magnesia-Carbon Bricks [J]. China's Refractories, 2007, 16(3): -. |
| [12] | ZHAO Shike, SHI Ying, HUANG Xiaoxian, GUO Jingkun,WU Jingyuan, HU Pei. Phase Composition and Thermal Expansion of CaO Stabilised ZrO2 Refractories [J]. China's Refractories, 2000, 9(4): -. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||