China's Refractories ›› 2022, Vol. 31 ›› Issue (2): 40-44.DOI: 10.19691/j.cnki.1004-4493.2022.02.008
Previous Articles Next Articles
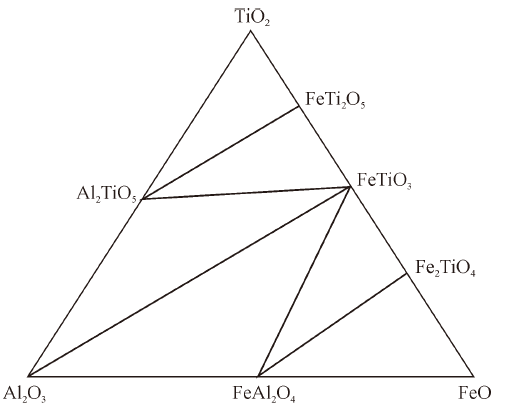
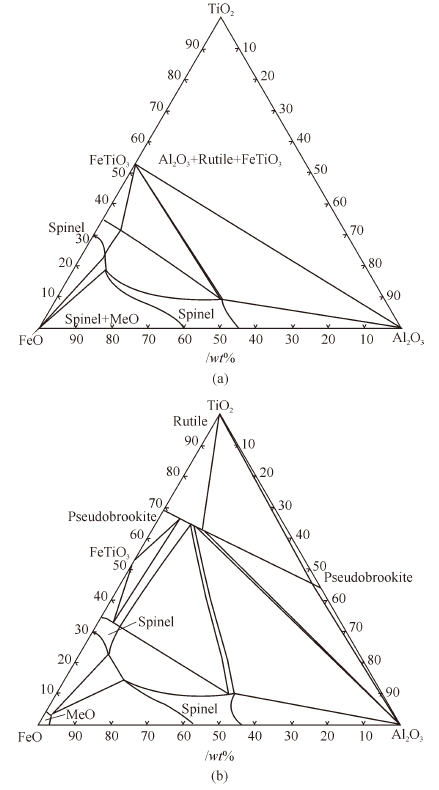
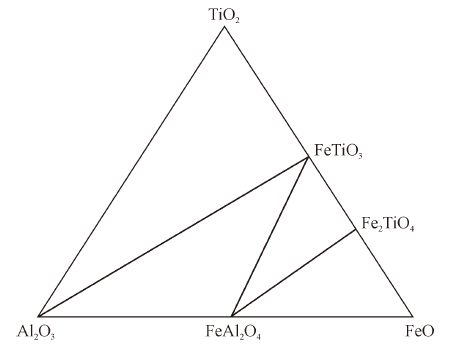
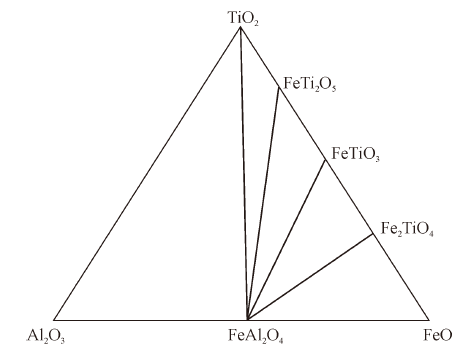
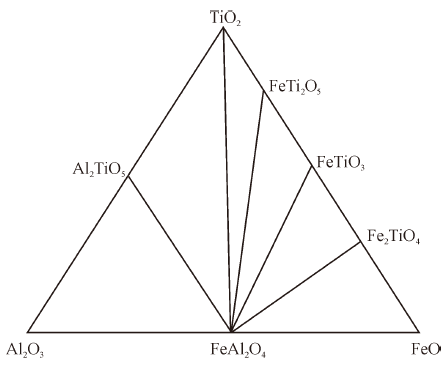
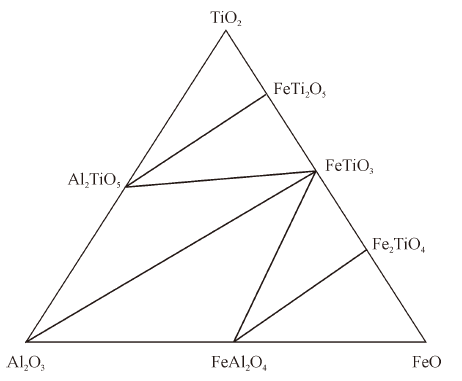
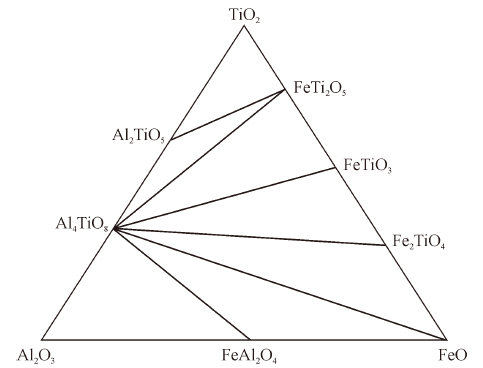
Thermodynamics of Phase Equilibria in FeO-TiO2-Al2O3 System
Oksana M. BORYSENKO*( ), Sergey M. LOGVINKOV, Galina M. SHABANOVA, Andrii A. IVASHURA, Alla M. KOROHODSKA
), Sergey M. LOGVINKOV, Galina M. SHABANOVA, Andrii A. IVASHURA, Alla M. KOROHODSKA
- Kharkiv Polytechnic Institute, National Technical University, Kharkiv 61002, Ukraine